Abstract
Tissue hypoxia secondary to profound anemia can lead to end organ damage with irreversible morbidity and mortality. Patients may choose not to receive blood products for religious reasons or may be unable to receive blood products due to alloimmunization. Artificial blood products have been explored as options for an alternative for blood transfusions, and prior synthetic oxygen carriers have been associated with an increased risk of myocardial infarction and stroke. Sanguinate is purified bovine hemoglobin (Hgb) that has been pegylated and combined with carbon monoxide which may suppress vasoconstriction and provide anti-inflammatory effects. The bovine hemoglobin then carries oxygen for release to areas with a low partial pressure of oxygen. Administration to 29 patients under FDA emergency provisions showed clinical improvements in one or more conditions in 86% of patients and no serious adverse events. Increases in blood pressure of 10-20 mmHg were seen with prior Sanguinate infusions presumably due to the colloid osmotic pressure of the pegylated protein solution. Some of the patients with elevated blood pressures developed fluid overload and increased troponin levels, especially the patients with end stage renal disease. Further efficacy and safety data are needed.
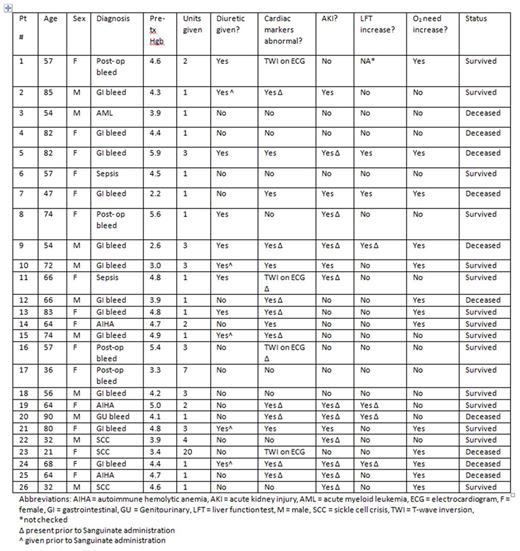
From November 2015 through April 2016, Sanguinate was prophylactically administered to adults with profound anemia on 26 occasions at Pennsylvania Hospital with the goal of preventing hypoxic events. Inclusion criteria included: age > 18, a Hgb < 5 g/dL or a Hgb < 7 g/dL with a 5 g/dL Hgb decrease within 7 days, refusal of blood products or an inability to receive blood products due to compatibility, willingness to receive supplemental iron therapy and erythropoietic- stimulating agents, ability to obtain consent, and investigator determination the patient would be an appropriate candidate for Sanguinate. The primary objective was to demonstrate the safety of Sanguinate infusion in patients with acute severe anemia who cannot receive RBC transfusion. The primary endpoint was the number and severity of adverse events within 24 hours of each Sanguinate infusion.
Average patient age was 62 years (range 21-90.) Average pre- treatment Hgb was 4.3 g/dL (range 2.2- 5.9 g/dL.) Overall survival was 61.5%. Table 1 contains a full list of patient characteristics. No serious adverse events, defined as death, a life-threatening adverse event, disruption in ability to conduct normal life functions, or prolongation of hospitalization, were attributed to Sanguinate; ten deaths were attributed to end organ damage related to profound anemia. Of the patients who survived, there were no long-term deficits, including injuries secondary to hypoxia, observed. Sanguinate administration before irreversible hypoxic events has the potential to bridge episodes of acute anemia in patients for whom blood is not an option and may address an unmet medical need with the potential to improve outcomes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal